China-based biopharmaceutical firm BeiGene (BGNE) has received approval for its anti-PD-1 antibody tislelizumab from the China National Medical Products Administration (NMPA) for the first-line treatment of patients with advanced non-squamous non-small cell lung cancer (NSCLC).
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
The NMPA has also given conditional approval for treating hepatocellular carcinoma (HCC) in patients who have previously received at least one systemic therapy. BeiGene specializes in developing immuno-oncology drugs for treating cancer.
President, Chief Operating Officer, and General Manager of China at BeiGene, Xiaobin Wu, said, “With today’s approvals, tislelizumab is now available in China in five indications covering lung, liver, bladder, and lymphoma…We hope to make tislelizumab available broadly in China through our science-based commercial team and globally through our collaboration with Novartis (NVS), in furtherance of our goal of expanding access to innovative, quality cancer treatments for more people worldwide.”
Tislelizumab has been approved for the first-line treatment of advanced non-squamous NSCLC based on results from a Phase 3 clinical trial (NCT03663205). In this trial, patients with stage IIIB or stage IV non-squamous NSCLC were administered tislelizumab together with pemetrexed and platinum chemotherapy. The progress of these patients was compared to patients who were given pemetrexed and platinum alone.
Tislelizumab’s conditional approval for HCC was based on the results of a single-arm, open-label, multicenter, global pivotal Phase 2 clinical trial (NCT03419897), which included 249 patients from eight regions and countries across Asia and Europe. (See BeiGene stock charts on TipRanks)
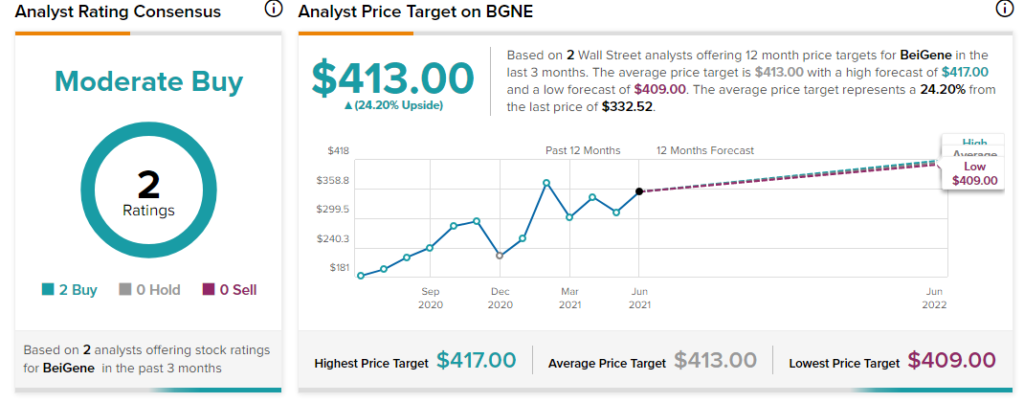
Morgan Stanley analyst Matthew Harrison recently maintained a Buy rating on the stock and increased the price target from $357 to $409 (23% upside potential).
Harrison believes that detailed ALPINE data announced during the virtual 25th Annual European Hematology Association (EHA) Congress could position Brukinsa to be the agent of choice in CLL (chronic lymphocytic leukemia), which could lead to significant upward consensus revisions in expected sales. “We now believe U.S. sales could achieve at least ~$2 billion in revenues and have raised our peak sales,” Harrison added.
Overall, the stock has a Moderate Buy rating based on 2 Buys. The average BeiGene analyst price target of $413 implies 24.2% upside potential from current levels. The company’s shares have gained 77.4% over the past year.
Related News:
Nikola Invests $50M in Clean Hydrogen Push
PerkinElmer to Acquire SIRION Biotech
Ford to Supply Parts for U.S. Postal’s New Delivery Vehicles – Report









