Immunic (IMUX), a clinical-stage biotechnology company, is developing a small-molecule oral medicine for the treatment of Multiple Sclerosis (MS). Its lead drug candidate, Vidofludimus Calcium (IMU-838), has displayed therapeutic efficacy and superior safety and tolerability for patients suffering from MS.
Recently, Immunic presented updated data from clinical and preclinical trials for Vidofludimus Calcium at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Let’s discuss the key takeaways from the conference.
- Serum Neurofilament Light Chain (Nfl) Levels – In the Phase 2 interim analysis of the CALLIPER trial, the drug showed clear differentiation from placebo in consistently reducing the Nfl levels across all age and disability levels for all progressive MS and its subtypes. In 203 patients, serum Nfl levels were reduced by 22.4% after 24 weeks of Vidofludimus Calcium treatment.
- Reducing Fatigue in MS patients and post-COVID Syndrome (PCS) – Clinical signals have proven that fatigue in PCS and MS patients could be related to the reactivation of the Epstein-Barr Virus (EBV). Immunic’s IMU-838 has shown positive signs of preventing reactivation of the EBV and the company is striving to prove that Vidofludimus Calcium has the potential to reduce fatigue in MS patients in the ongoing CALLIPER trial and Phase 3 ENSURE trials.
- Effect on Nurr1 in Preclinical Models – Vidofludimus calcium possesses potential neuroprotective functions, one of which is its effect on Nurr1 (nuclear receptor related 1) activator. The drug has shown direct neuroprotective effects of improving neuronal survival and indirect effects of reducing neurotoxic activation of microglia cells. Preclinical trials showed that mice receiving the drug exhibited higher levels of brain-derived neurotrophic factor (BDNF) in the blood and enhanced expression of Nurr1 and its target gene in the central nervous system. Activation of Nurr1 helps in halting neurodegeneration and disability progression in MS patients.
- Development of Pathogenic Peripheral T Helper Cells – In preclinical trials, the drug reduced or prevented the development of infiltrating T Helper Cells in the spinal cord and in the periphery. This helps in reducing disease severity and prevents disease development in MS patients.
Interestingly, analyst William Wood of B. Riley Financial attended the conference and came back with positive views on the potential benefits of Vidofludimus Calcium as compared to other late-stage MS treatments. Let’s take a quick view of Wood’s views on the drug.
- Vidofludimus Calcium’s Competitive Advantage Compared to Peers – The drug uses brain volume loss (BVL) as a primary endpoint in the Phase II CALLIPER trials in PMS, with initial indications showing that the drug reduces brain atrophy. Meanwhile, other late-stage drugs have not shown significant improvement in BVL, hence giving an edge to Immunic.
- Safety tolerability study of Vidofludimus – Vidofludimus calcium has displayed greater safety and tolerability profile as compared to peers, especially in terms of increasing ALT (Alanine Aminotransferase) levels as well as bilirubin increase. Some of the other drugs which led to elevated ALT levels in patients had to discontinue treatment. ALT levels are tested to see the impact and damage done to the liver.
- General View on Vidofludimus Calcium – Overall, Wood came back with positive views on the drug’s potential clinical benefits that were displayed via the four key takeaways discussed above. These include improving Nfl levels, neuroprotective benefits, halting reactivation of EBV and thus reducing fatigue, as well as updated promising data from preclinical tests. The analyst is looking for further differentiation and clarity on Vidofludimus Calcium’s higher clinical benefits from the upcoming Phase III RMS study futility analysis and the readout of the Phase II PMS top-line data in April 2025.
- Wood’s View on IMUX Stock – Following the conference, Wood reiterated a Buy rating on IMUX stock with a price target of $6, which implies 263.6% upside potential from current levels.
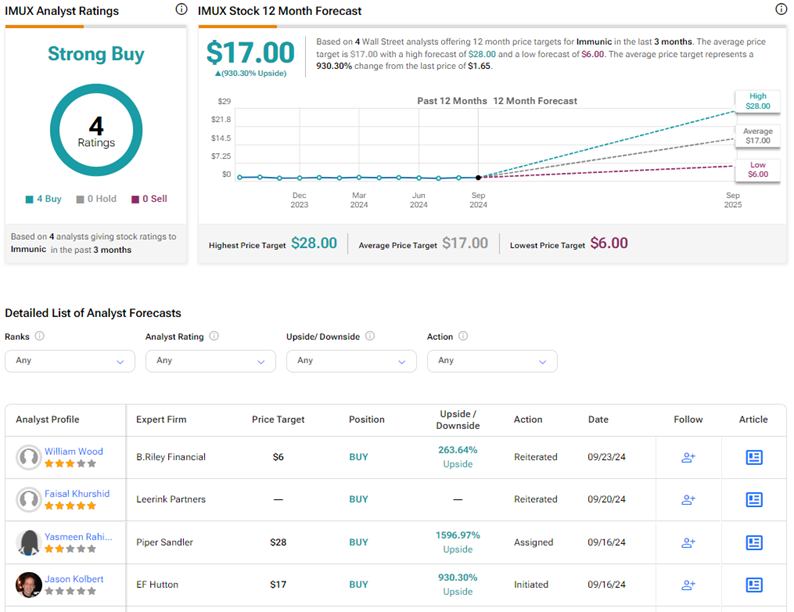
Is IMUX a Good Stock to Buy?
With four unanimous Buy ratings on TipRanks, IMUX stock commands a Strong Buy consensus rating. Also, the average Immunic price target of $17 implies 930.3% upside potential from current levels. Year-to-date, IMUX stock has gained 10%.
This article was written in partnership with Immunic. TipRanks may be compensated for its publication.
















