Shares of Avrobio plunged 17.6% on Friday after the clinical-stage gene therapy company kicked off a secondary offering of 5 million common shares. The offering is anticipated to close on Nov. 24.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Avrobio (AVRO) has priced the offering at $15 per share, 7.2% higher than the stock’s closing price of $13.99 on Nov. 20. According to the company, the gross proceeds from the offering would be approximately $75 million.
The biotechnology company stated that the net proceeds from the offering will be used “to fund its current programs in Fabry disease, cystinosis, Gaucher disease type 1, Hunter syndrome, Pompe disease and Gaucher disease type 3, fund external and internal manufacturing and process development activities and fund research and development activities that relate to its current and future clinical and preclinical activities, including the cost of research and development personnel.” (See AVRO stock analysis on TipRanks)
Morgan Stanley, Cowen, Wells Fargo and Barclays are the joint book-running managers of the offering. Avrobio has provided a 30-day option to underwriters to purchase an additional 0.75 million shares at the offering price.
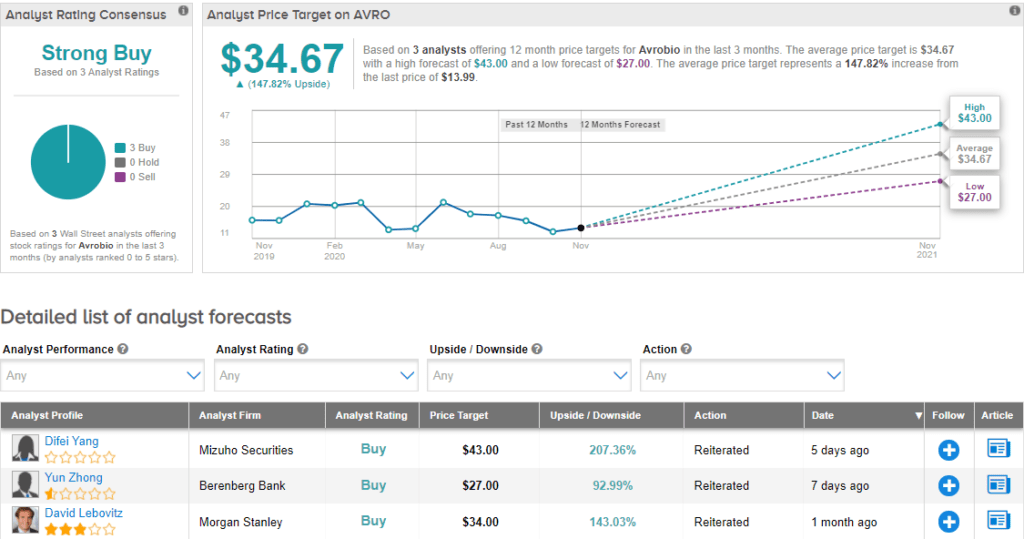
On Nov. 18, Mizuho Securities analyst Difei Yang raised the stock’s price target to $43 (207.4% upside potential) from $35 and reiterated a Buy rating. In a note to investors, Yang said that she lifted the price target “on greater probability of success in two clinical programs (Cystinosis and Gaucher).” The analyst continues to see “significant value” in AVRO.
Currently, the Street has a bullish outlook on the stock with a Strong Buy analyst consensus. The average price target stands at $34.67 and implies upside potential of about 147.8% to current levels. Shares are down by about 30.5% year-to-date.
Related News:
Norwegian Cruise Prices Offering at $20.80 Per Share; Stock Down 62% YTD
Eiger Pops 12% On FDA Approval For First Hutchinson-Gilford Progeria Treatment
FDA To Review Pfizer-BioNTech Covid-19 Vaccine On Dec. 10









