Athenex announced on Tuesday that the US Food and Drug Administration (FDA) has accepted the new drug application (NDA) for its oral paclitaxel treatment of metastatic breast cancer sending shares up 12%.
Athenex (ATNX) shares soared to $12.82 in Tuesday’s morning trading. The biotech company disclosed that the NDA for its treatment of metastatic breast cancer has also been granted priority review by the FDA.
The FDA grants priority review to applications for potential drugs that, if approved, would be significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions when compared to standard applications, the company said. The FDA has scheduled a decision deadline for February 28, 2021. Additionally, the FDA has communicated that it is not currently planning to hold an advisory committee meeting to discuss the application.
“We continue to finalize our commercial preparations to ensure a successful launch of oral paclitaxel, if approved,” said Athenex CEO Johnson Lau. “We see oral paclitaxel as a potentially important alternative to intravenous (IV) infusions, especially during the current pandemic, as it may allow cancer patients to take the oral chemotherapy at home. We believe the oral paclitaxel program validates our broader Orascovery platform, and we are committed to applying the technology to convert other IV chemotherapies into oral agents.”
Oral paclitaxel NDA submission is supported by data from a single pivotal Phase III for the treatment of metastatic breast cancer. The study is a randomized, controlled clinical trial designed to compare the safety and efficacy of oral paclitaxel monotherapy versus IV paclitaxel monotherapy. It achieved its primary endpoint showing statistically significant improvement in overall response rate, along with a lower neuropathy, for oral paclitaxel compared to IV paclitaxel, the company said.
The orascovery platform was initially developed by Hanmi Pharmaceuticals and licensed exclusively to Athenex for all major worldwide territories except Korea, which is retained by Hanmi.
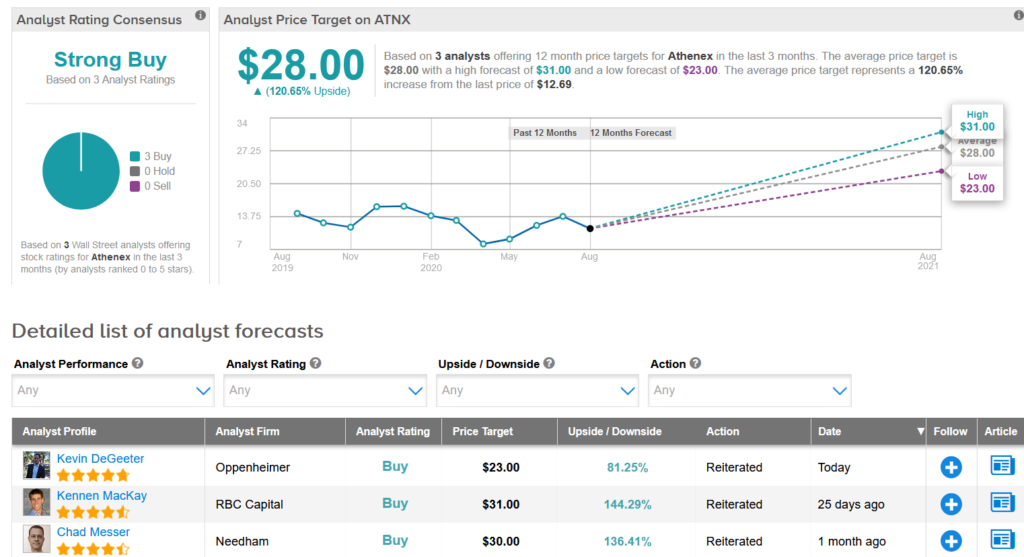
ATNX is down 17% so far this year, with the $28 average analyst price target suggesting that there is room for a whopping 121% upside potential in the shares over the coming year.
Oppenheimer analyst Kevin DeGeeter last month maintained a Buy rating on the stock with a $23 price target, saying that he “continues to view ATNX’s portfolio of oral cancer drugs as well positioned to address evolving SOC in response to COVID-19”.
With 3 unanimous Buy ratings, the stock scores a Strong Buy analyst consensus. (See Athenex stock analysis on TipRanks)
Related News:
Sanofi Says Kevzara Drug Fails To Meet Endpoints in Covid-19 Study
AstraZeneca Kicks Off US Late-Stage Trial Of Covid-19 Vaccine Candidate
T2 Bioystems Spikes 19% On FDA Nod For Covid-19 Molecular Test
















