AstraZeneca Plc (AZN) announced on Thursday that the Food and Drug Administration (FDA) has accepted a supplemental New Drug Application (sNDA) and granted a priority review for its heart treatment Brilinta.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The U.S. regulator granted priority review for the drugmaker’s Brilinta(ticagrelor) as a treatment for the reduction of subsequent stroke in patients who experienced an acute ischemic stroke or transient ischemic attack (TIA). The FDA action date for the supplemental application, is scheduled for the fourth quarter of 2020.
The sNDA was based on results from the Phase III THALES trial, which showed that the treatment of aspirin in combination with Brilinta90mg dosage used twice daily for 30 days resulted in a statistically significant and clinically meaningful reduction in the risk of the primary composite endpoint of stroke and death, compared to aspirin alone.The results were in line with the known safety profile of Brilinta.
“Patients who have had an acute ischaemic stroke or transient ischemic attack are at high risk of experiencing a subsequent stroke, which may be disabling or fatal,” said Mene Pangalos, Executive VP, BioPharmaceuticals R&D. “Today’s priority review reflects Brilinta’s potential as a much-needed treatment option to reduce the rate of subsequent stroke for these patients and we look forward to working with the FDA to make Brilinta available as soon as possible.”
Brilinta is approved in more than 110 countries for the prevention of atherothrombotic events in adult patients with acute coronary syndrome (ACS) and in more than 70 countries for the secondary prevention of cardiovascular events among high-risk patients who have experienced a heart attack.
Stroke is the second leading cause of death worldwide, with 6.2 million stroke-related deaths in 2017, of which 2.7 million were due to ischaemic stroke.
In May 2020, the FDA approved a label update for Brilinta in the US to include the risk reduction of a first heart attack or stroke in high-risk patients with coronary artery disease.
AstraZeneca shares have jumped 43% since mid-March as the company joined the list of companies engaged in the development of a potential coronavirus vaccine. The stock rose 1.4% to close at $54.19 on Wednesday.
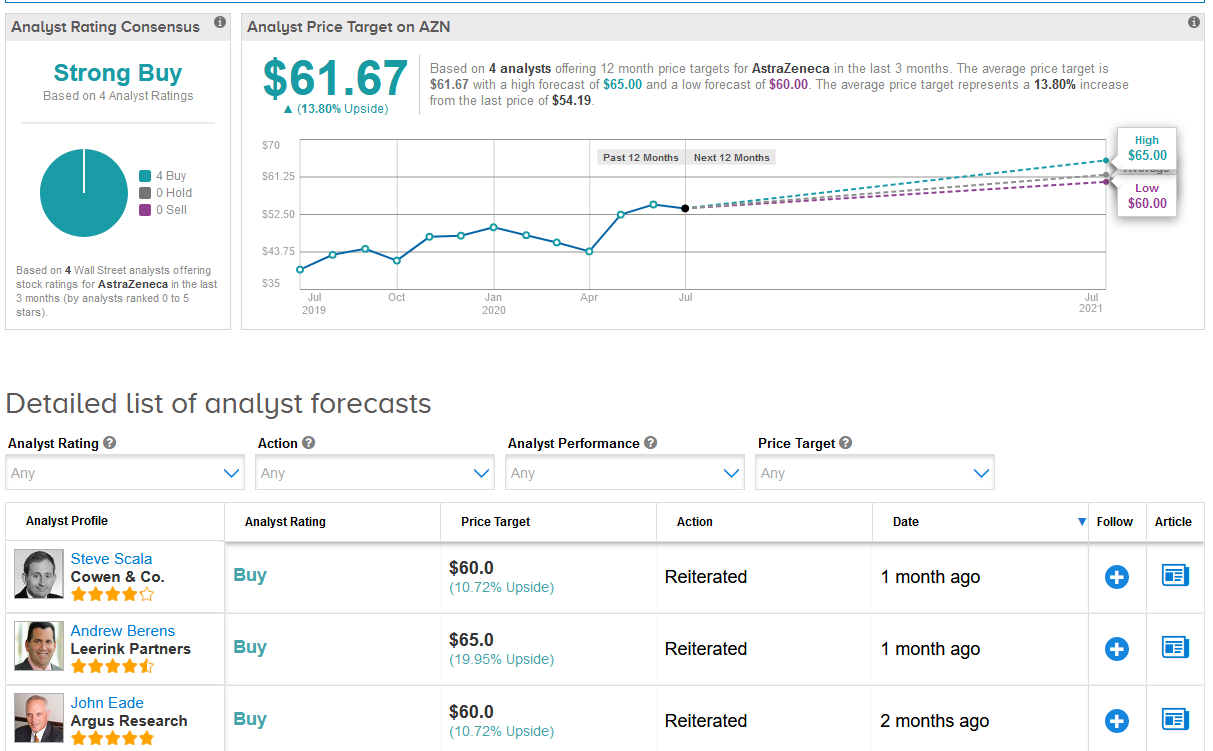
Despite the recent rally, the $61.67 average analyst price target still puts the upside potential at 14% in the coming 12 months. (See AstraZeneca stock analysis on TipRanks)
Overall, the stock scores a Strong Buy consensus from the analyst community based on 4 unanimous Buy ratings.
Related News:
AstraZeneca-Merck Pancreatic Cancer Drug Wins European Approval
Novavax Spikes 42% Pre-Market On $1.6B U.S. Funding For Covid-19 Candidate
Corvus Shoots Up 115% On Start Of Novel Immunotherapy Study In Covid-19 Patients