AstraZeneca announced that the US regulator has approved its heart treatment Brilinta to be used to reduce the risk of an attack in patients with acute ischemic stroke.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The decision by the US Food and Drug Administration (FDA) extends Brilinta’s treatment beyond cardio-vascular disease to patients with mild-to-moderate stroke and is also intended to reduce the risk of recurrent strokes.
The FDA approval was based on positive results from AstraZeneca’s (AZN) Phase 3 trial that showed that the use of aspirin with a 90mg dosage of Brilinta “significantly” reduced the rate of the composite of stroke and death compared to aspirin alone in patients with acute ischemic stroke or high-risk transient ischemic attack (TIA).
The move comes after the FDA approved priority view designation for the drug in July this year. An ischemic stroke is caused by a blockage cutting off the blood supply to a region of the brain. A transient ischemic attack, is a temporary blockage of the blood supply to a region of the brain, resulting in symptoms only lasting for a short amount of time. In the US, every 4 minutes, someone dies of a stroke and about 1 in 4 strokes are recurrent.
“In the US, someone has a stroke every 40 seconds and the impact on a person’s life can be truly devastating,” said AstraZeneca’s Mene Pangalos. “Brilinta is a well-established medicine across patients with coronary artery disease and with today’s approval, we can now expand its potential to patients with an acute ischemic stroke or transient ischemic attack.”
More specifically, the Phase 3 trial demonstrated that the Brilinta 90mg dosage used twice daily and taken with daily aspirin for 30 days, reduced the rate of the primary composite endpoint of stroke and death by 17%, compared to aspirin alone in patients with an acute ischemic stroke or TIA. “This was a statistically significant and clinically meaningful reduction,” AstraZeneca said.
Regulatory submissions to expand the approved indication are also under regulatory review in China and in the EU where the medicine’s name is Brilique, the drugmaker added.
AZN shares are up 10.7% over the past 5 days making up most of this year’s advance. (See AstraZeneca stock analysis on TipRanks)
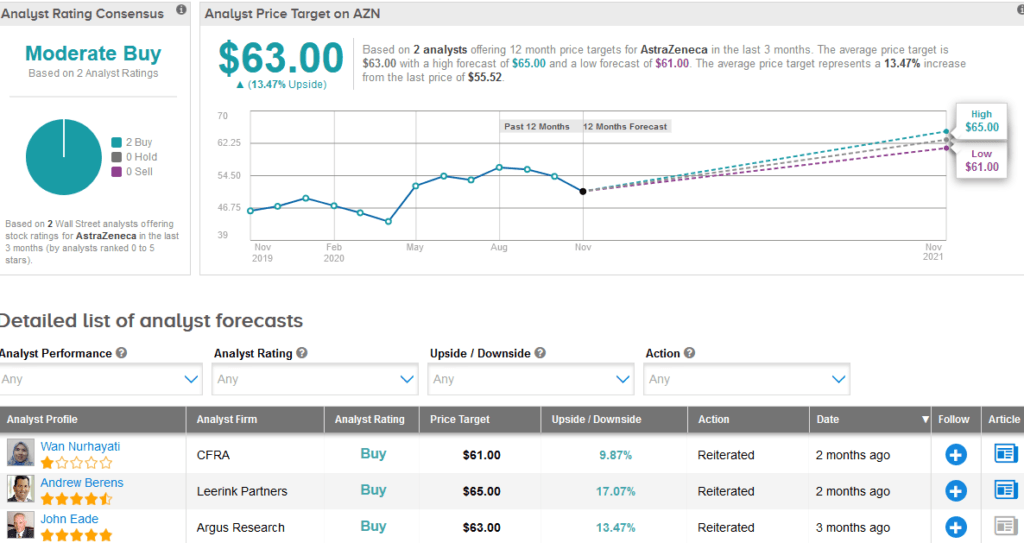
CFRA analyst Wan Nurhayati recently reiterated a Buy rating on the stock with a $61 price target (9.9% upside potential) as she believes that the company’s new medicines will help grow revenue in the high single-digit percentage range in 2020.
Nurhayati doesn’t expect AstraZeneca’s Covid-19 vaccine candidate to be a major profit driver, as the drugmaker is prioritizing affordable distribution.
Overall, AZN scores a Strong Buy analyst consensus with 2 unanimous Buy ratings. Meanwhile, the $63 average analyst price target puts the upside potential at about 14% in the coming 12 months.
Related News:
Hologic Quarterly Profit Triples On Covid-19 Test Sales; Shares Rise
Biogen Rockets 44% On “Substantial Evidence” For Alzheimer’s Drug Approval
J&J Strikes Covid-19 Vaccine Manufacturing Deal With Aspen










