AstraZeneca will start to supply the European Union with an additional 9 million doses of its vaccine after Europe approved its use to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older.
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
This marks another approval for AstraZeneca’s (AZN) vaccine after getting the regulatory nod from the UK. The European Medicines Agency (EMA) granted a so-called conditional marketing authorisation (CMA) based on positive opinion on data from a rolling review of the primary analysis of the Phase 3 study led by the University of Oxford.
Following the review, the Committee for Medicinal Products for Human Use (CHMP) of the EMA recommends two doses of AstraZeneca’s COVID-19 vaccine, to be deployed at a four- to 12-week interval in individuals aged 18 years and older. The dosing regimen demonstrated to be safe and effective in preventing symptomatic COVID-19, with no severe cases and no hospitalisations more than 14 days after the second dose, the CHMP concluded.
“AstraZeneca will deliver 9 million additional doses in the first quarter (40 million in total) compared to last week’s offer & will start deliveries one week earlier than scheduled,” European Commission president Ursula Von der Leyen wrote in a Twitter post on Jan. 31. “The company will also expand its manufacturing capacity in Europe.”
AstraZeneca is also conducting a large trial in the US and globally. As a result, Oxford University and AstraZeneca expect to enroll up to 60,000 participants globally. AstraZeneca is scheduled to supply up to three billion doses of the vaccine in 2021 globally, pending regulatory approvals.
“Today’s approval underscores the value of AstraZeneca’s COVID-19 vaccine, which is not only effective and well tolerated, but also easy to administer and, importantly, protects fully against severe disease and hospitalisations,” said AstraZeneca CEO Pascal Soriot.
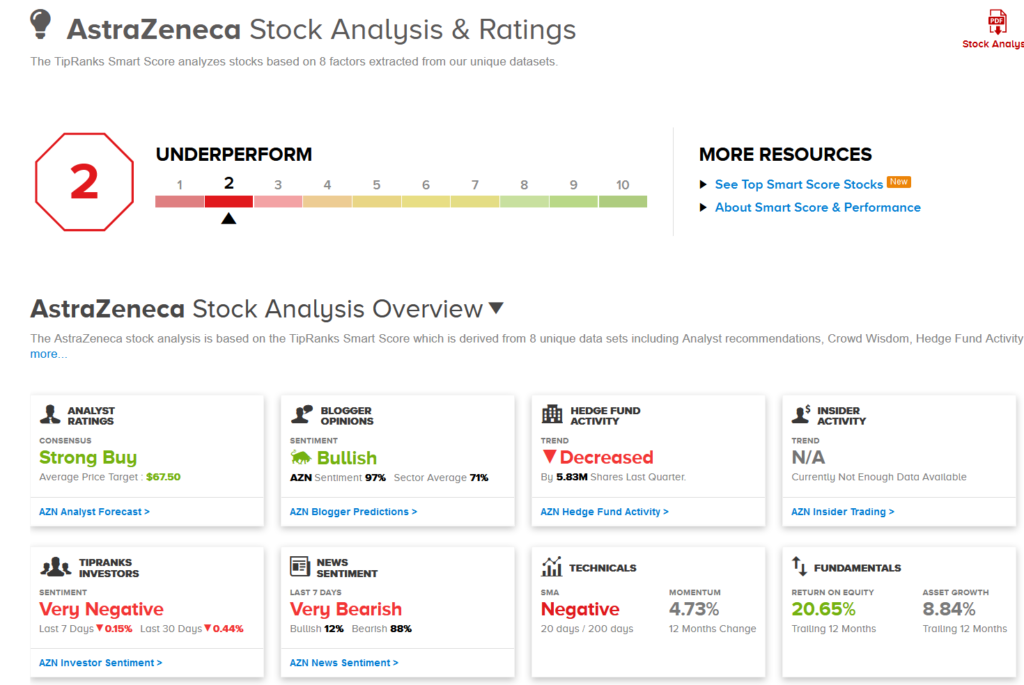
AZN shares have declined 6.3% over the past five days and are up 4.3% over the past year. (See AstraZeneca stock analysis on TipRanks). That’s with a Strong Buy consensus rating backed by 6 Buy ratings versus only 1 Hold rating.
What’s more, the average analyst price target stands at $67.50, which puts the upside potential at a promising 33% in the coming 12 months.
Following the vaccine’s UK approval, AlphaValue analyst Amandeep Goyal upgraded the stock to Buy from Sell as he argues that “sentiment around Astra’s share price should improve finally.”
Goyal also raised AZN’s premium on peer-based multiples to 40% from 35% to account for prospective incremental cash flows from the Alexion takeover.
Meanwhile, AZN gets a 2 out of 10 on TipRanks’ Smart Score system , which implies that the stock is likely to underperform market expectations.
Related News:
Microsoft’s Cloud Services Fuel 2Q Sales Beat; Shares Rise
Starbucks’ Profit Outlook Disappoints; Shares Fall
Texas Instruments’ 4Q Sales Top Estimates; Street Says Hold









