U.S. Food and Drug Administration’s independent advisors committee collectively proposed the use of AstraZeneca (NASDAQ:AZN) and Sanofi’s (NASDAQ:SNY) monoclonal antibody Nirsevimab for protecting infants from respiratory syncytial virus (RSV) infections, a dominant cause of hospitalization among newborns.
The approval was with a 21-0 panel vote on Thursday. FDA did not identify any safety concerns in its review of Nirsevimab. While AstraZeneca makes Nirsevimab, it would be marketed by Sanofi. The drug is already approved in Canada, Europe, and the U.K.
Separately, advisors with a 19-2 panel vote also recommended the use of the drug in children up to 2 years old, who remain vulnerable to the virus in their second RSV season.
If Nirsevimab wins the FDA’s final approval, the antibody will become the first medical intervention available in the U.S. that could protect infants from RSV. A final decision by the FDA is expected in Q3.
An FDA review indicates that Nirsevimab was up to 75% effective at preventing lower respiratory tract infections that required medical attention and 78% effective at preventing hospitalizations.
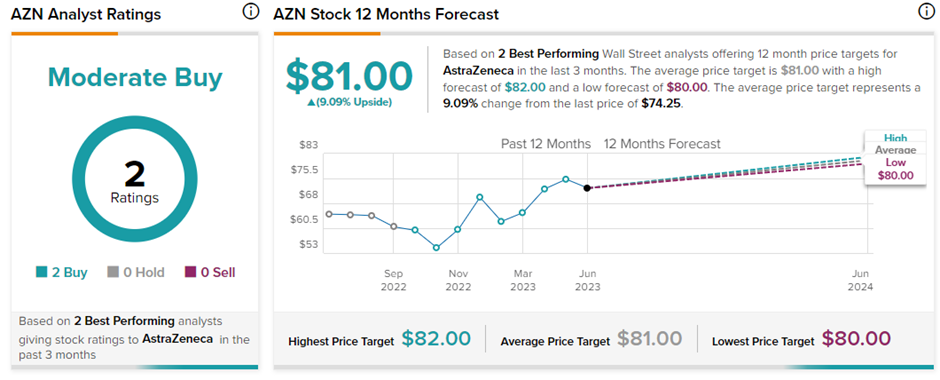
Is AZN Stock a Good Buy?
In the past two weeks, two top Wall Street Analysts covering AZN stock reaffirmed their Buy ratings, bringing the consensus rating to a Moderate Buy. The average price target set at $81 indicates a 9.09% upside potential.