The U.S. Food and Drug Administration (FDA) approved a treatment developed by AstraZeneca (AZN) and Merck Co. (MRK) for advanced ovarian cancer.
The FDA approved the ovarian drug Lynparza to be used in combination with bevacizumab as a first-line maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer. Patients will be selected for therapy based on an FDA-approved companion diagnostic for Lynparza.
Lynparza is a first-in-class PARP inhibitor, which is a targeted treatment to potentially exploit DNA damage response (DDR) pathway deficiencies, such as so-called BRCA mutations to kill cancer cells. It is being tested in a range of tumor types. Ovarian cancer is the fifth most common cause of death from cancer in women in the U.S. This year, it is estimated that more than 21,000 women will be diagnosed with ovarian cancer and nearly 14,000 women will die of the disease.
The nod by the U.S. regulator followed a biomarker subgroup study of 387 patients with positive tumors from the Phase 3 PAOLA-1 trial, which showed that the Lynparza drug in combination with bevacizumab reduced the risk of disease progression or death by 67%. It improved progression-free survival (PFS) to a median of 37.2 months vs. 17.7 months with bevacizumab alone in patients with advanced ovarian cancer.
“Advances in understanding the role of biomarkers and PARP inhibition have fundamentally changed how physicians treat this aggressive type of cancer,” said Dr. Roy Baynes, senior vice president and chief medical officer at Merck Research Laboratories. “Today’s approval based on the PAOLA-1 trial highlights the importance of homologous recombination deficiency (HRD) testing at diagnosis to identify those who may benefit from Lynparza in combination with bevacizumab as a first-line maintenance treatment.”
AstraZeneca and Merck said they are also seeking to get approval by the European Union, Japan and other countries for Lynparza’s use in combination with bevacizumab as a first-line maintenance treatment for patients with advanced ovarian cancer.
Mara Goldstein, analyst at Mizuho Securities reiterated Merck’s Buy rating with a $100 price target.
“The likelihood that MRK’s business rebounds in 2H20 with the easing of stay-at-home restrictions is high, in our view, and drives our support of the shares,” Goldstein wrote in a note to investors.
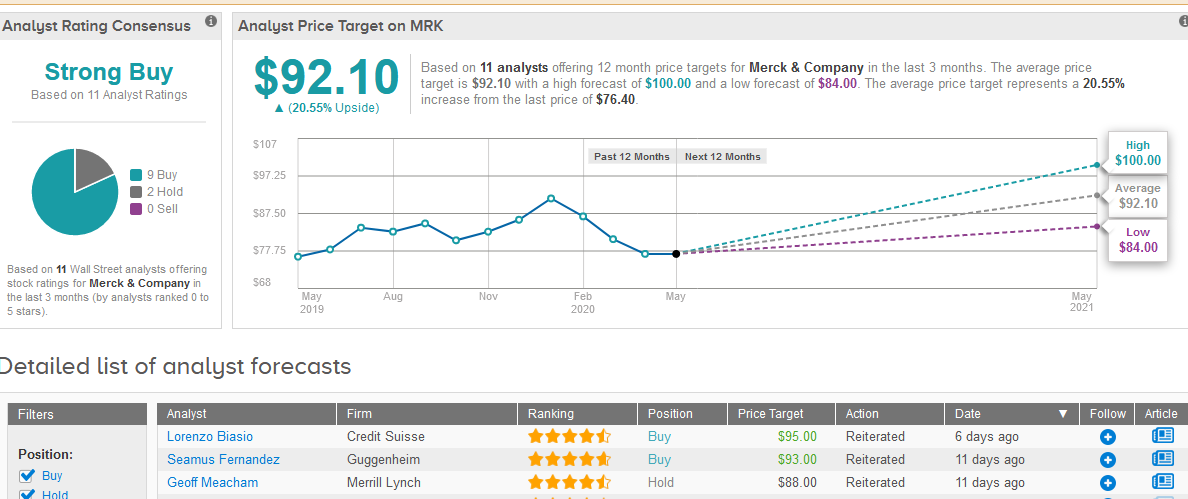
TipRanks data shows that 9 out of the 11 Wall Street analysts covering the stock in the past three months have a Buy on the stock. The remainder say Hold adding up to Strong Buy consensus rating. The $92.10 average price target suggests 21% upside potential in the shares in the coming 12 months. (See Merck stock analysis on TipRanks).
Related News:
Quidel’s Rapid Covid-19 Antigen Test Scores Emergency FDA Approval
Tesla’s Elon Musk Takes Legal Action to Fight Reopening of California Car Plant
Apple to Reopen Some U.S. Stores Next Week