AstraZeneca Plc (AZN) and Daiichi Sankyo Co Ltd. said Monday that their jointly developed drug Enhertu used for the treatment of advanced gastric cancer has been granted Breakthrough Therapy Designation (BTD) status from the U.S. Food and Drug Administration (FDA).
The BTD status will allow the companies to accelerate the development, regulatory review and market entry of Enhertu. The FDA awards the status for potential new medicines that are intended to treat a serious condition and address a significant unmet medical need. Enhertu is an antibody drug targeting HER2-positive cancer cells.
“Current therapy options are limited for patients with HER2-positive metastatic gastric cancer and for those who relapse, there are no approved HER2-targeted medicines,” said José Baselga, Executive Vice President, R&D Oncology. “We look forward to working with the FDA to further explore the potential of Enhertuto become an important new treatment and the first antibody drug conjugate for this devastating disease.”
Gastric cancer is the fifth most common cancer worldwide and the third leading cause of cancer mortality. In the US, it is estimated that 27,600 new cases of gastric cancer will be diagnosed in 2020 and more than 11,000 people will die from the disease. About one in five gastric cancers are considered HER2 positive.
The FDA granted the BTD status based on Phase I trial and Phase II trial data. Patients treated with Enhertu demonstrated a statistically significant and clinically meaningful improvement in objective response rate (ORR), the primary endpoint, and overall survival (OS), a key secondary endpoint, versus patients treated with chemotherapy.
Enhertu is already approved in the US and Japan for the treatment of adult patients with metastatic HER2-positive breast cancer.
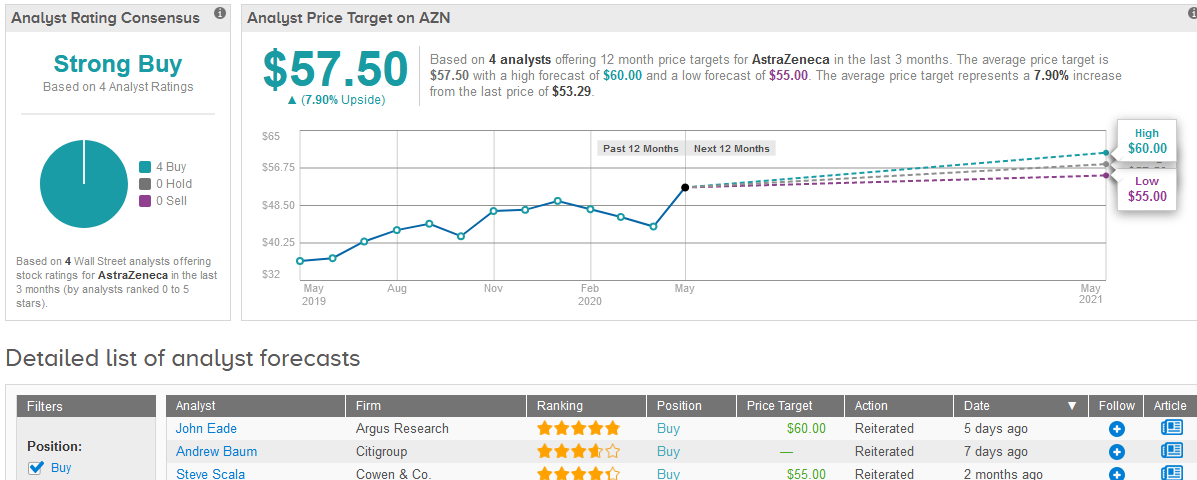
The drugmaker’s shares rose 1.1% to $53.29 on Friday after rallying 29% in the past 2 months.
TipRanks data shows that Wall Street analysts have a bullish outlook on the stock as 4 have Buys adding up to a Strong Buy consensus rating. The $57.50 average price target puts the upside potential for the shares in the coming 12 months at 7.9%. (See AstraZeneca stock analysis on TipRanks).
Related News:
AstraZeneca-Merck Ovarian Cancer Treatment Gets FDA Approval
Seres Therapeutics Reports Weak Earnings, But Significant Upside Lies Ahead
Eli Lilly Wins FDA Approval For Retevmo Lung, Thyroid Cancer Treatment