In the biotechnology world, clinical data readouts are make-or-break. Release favorable results, and rewards in the form of sky-high gains could be on tap, but if the data misses the mark, shares could also be catapulted, only in the opposite direction.
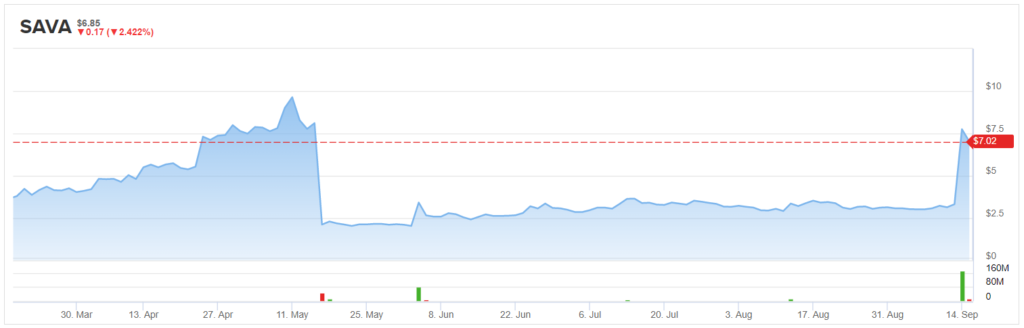
Cassava Sciences (SAVA) is a prime example of the former. On Monday, the company published the final results from the Phase 2b study of sumifilam (PTI-125), its small molecule drug that targets an altered form of filamin A (FLNA), in mild-moderate Alzheimer’s disease (AD). The data demonstrated statistically significant improvements in biomarkers of disease and cognition. In response, shares rounded out the day’s session up 133%.
Back in May, analysis of the data failed to show statistical significance. However, 5-star analyst Jason McCarthy, of Maxim Group, points out that “it was unclear at the time if there were anomalies in the data, particularly in the placebo group which caused the miss.” The reanalysis, which showed the therapy did in fact have a meaningful impact on the biomarkers associated with the disease, was in-line with what had been observed in the prior Phase 2a study.
Looking at the Phase 2a study, patients achieved a 100% responder rate with statistically significant decreases across key biomarkers. This included a 20% reduction in total tau, 34% in phosphorylated tau and 22% in Neurofilament light chain, among others. The Phase 2b trial was then designed using this data.
“While the trial missed its endpoint of showing significant differences between drug and control on markers of AD inflammation and other biomarkers of disease, it was noted that the data seemed to have significant variability not observed in the prior study which may in part have driven the stat-sig miss. How? Mainly in the placebo group biomarker data,” McCarthy commented.
Originally, SAVA only expected to see “small changes in the levels of biomarkers over 28 days, that biomarkers generally move in the same direction and biomarkers that move in tandem show robust statistical correlations.” However, the company actually saw “large dramatic swings in levels of biomarkers, random movement of biomarkers sometimes in opposite directions and uncorrelated changes in levels of biomarkers.” McCarthy noted, “Combined, it was believed by the company that there could be a potential problem with the data and that the data needed to be reanalyzed, which was reported on September 14.”
Going forward, the next step for SAVA will be to have an end-of-Phase 2 meeting with the FDA, which McCarthy believes will come in 2021 given the FDA’s calendar for the rest of 2020. It will also complete the ongoing open-label extension study of sumifilam. Then, SAVA will develop plans for the Phase 3 program.
Everything that SAVA has going for it convinced McCarthy to upgrade his rating from Hold to Buy. Along with the call, he attached a $14 price target, suggesting 104% upside potential. (To watch McCarthy’s track record, click here)
To find good ideas for healthcare stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analyst. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.
Questions or Comments about the article? Write to editor@tipranks.com