The markets have been volatile recently as economic uncertainty and tariff threats have rattled investors. Yet, despite the concerns, Goldman Sachs’ chief US equity strategist David Kostin sees reason for long-term optimism – and makes some concrete recommendations about where investors should put their funds to cash in.
Simply put, Kostin believes that healthcare stocks are the way to go in 2025. Healthcare makes up more than 17% of the total US economy, and it has a defensive tilt – even if times go sour, healthcare remains in demand. In addition, while healthcare stocks underperformed the S&P in the last two years, the sector is outperforming so far this year.
Summing up the situation and the outlook, Kostin writes, “We maintain our year-end S&P 500 price target of 6500. The equity market’s pricing of the economic growth outlook is now in the ballpark of our economists’ baseline economic growth forecasts, and the S&P 500 P/E of 21.5x is in line with our year-end S&P 500 P/E multiple forecast…”
“Within the equity market, we continue to recommend investors own the Health Care sector, which offers investors a defensive tilt at low valuations,” Kostin went on to add. “Health Care has outperformed the S&P 500 by 7 pp YTD but the median stock still trades at an 18% P/E discount to the S&P 500, nearly the largest valuation discount in recent decades.”
The stock analysts at Goldman are following this line and pulling the trigger on two healthcare stocks in particular. We’ve used the TipRanks database to look up the broader Wall Street view on both, and find out just why they’re compelling buys; here are the details.
Vaxcyte, Inc. (PCVX)
The first Goldman choice is Vaxcyte, a biotech researcher focused on developing new vaccines designed to target difficult-to-treat bacterial infections. The company is taking a novel approach to the development of anti-bacterial vaccines by using a combination of ‘advanced chemistry and modern synthetic techniques’ to create a cell-free protein synthesis platform. The goal is to rapidly design and engineer difficult-to-make proteins that can deliver strong immunological benefits – specifically, immune reactions against targeted bacterial infections.
The challenge here is overcoming the natural array of defenses that bacteria have evolved to cope with both immune responses and new drugs. Bacteria have various physical, biochemical, and immunological mechanisms, which they use to counteract natural immune system defenses or antibiotic drug agents; Vaxcyte is working to create new vaccines that act through channels that are novel to the bacterial world.
The company is using a cell-free approach to devise new modes of protein expression, and its proprietary cell-free development platform has allowed the company to design precise, site-specific conjugation agents that preserve essential immune epitopes while attacking bacterial targets. In short, the company is creating bacteria-specific vaccines that cause fewer adverse effects for the patient.
Currently, Vaxcyte has a vaccine candidate under development against four serious bacterial infections: pneumococcal disease, group A strep, periodontitis, and shigella. The latter three of those are serious bacterial diseases with large global health impacts. Group A strep causes approximately 800 million cases of illness worldwide every year; periodontitis causes serious gum and mouth disease and affects approximately 65 million adults in the US alone; and shigella infections result in some 600,000 deaths worldwide every year. Vaxcyte’s research programs on these tracks are currently at the preclinical level.
At the human trial stage, Vaxcyte has two vaccine candidates currently under study in the trial clinic. The more advanced of these candidates, VAX-31, is under study in two clinical lines, for the treatment of pneumococcal disease in adults over age 50 and in infants. The company has successfully completed a Phase 2 study on the adult track and is making preparations to initiate a Phase 3 study of VAX-31 in adults over 50 by the middle of this year. Topline safety, tolerability, and immunogenicity data are expected for release during 2026.
On the infant track, Vaxcyte has initiated the second and final stage of a Phase 2 study for VAX-31, based on successful earlier studies. Topline data on safety, tolerability, and immunogenicity are expected in mid-2026, to be followed nine months later by data on the booster dose.
The company’s second pneumococcal disease vaccine study focuses on VAX-24, a vaccine under investigation in infants. Vaxcyte’s VAX-24 study is at Phase 2, and the company plans to release the topline data on safety, tolerability, and immunogenicity by the end of the current quarter. Booster data will follow by the end of this year.
These clinical studies don’t come cheap, but Vaxcyte has deep pockets. The company reported having $3.1 billion in cash and other liquid assets as of December 31, 2024.
It’s the combination of the platform tech and the strong potential of VAX-31 that has caught the eye of Goldman Sachs analyst Chris Shibutani. He writes of this company, “We view PCVX’s platform technology as differentiated in bacterial infection disease prevention, with promising potential to address large TAM markets with unmet need (e.g. shigella, periodontitis, Strep A), and think VAX-31 is a highly attractive target to large pharmaceutical players with established commercial presence in PCV markets… We see Vaxcyte as being well positioned to succeed in Phase 3 studies and, if approved, become the pneumococcal conjugate vaccine (PCV) of choice in a category that is expected to produce ~$8bn of annual global sales in 2024, and grow to over $10bn by 2030 – a market that has historically seen the leading vaccine take the bulk of market share.”
These comments support Shibutani’s Buy rating on the stock, while his $138 price target implies a one-year gain of 87% for the shares. (To watch Shibutani’s track record, click here)
PCVX stock has 7 recent analyst reviews on record, and they are all positive – for a unanimous Strong Buy consensus rating. The stock is priced at $73.89, and its $149.43 average price target suggests an upside this year of 102%. (See PCVX stock forecast)

Madrigal Pharmaceuticals (MDGL)
Goldman’s next pick is Madrigal Pharmaceuticals, a biotech company zeroing in on NASH (non-alcoholic steatohepatitis) – a progressive liver disease with serious consequences. NASH triggers fat buildup, inflammation, and cellular damage in the liver, potentially leading to cirrhosis, liver failure, or even cancer. Often linked to diabetes, this condition poses a growing health challenge, making Madrigal’s focus all the more critical.
We should note that Madrigal is a biotech company that has hit the jackpot – in March of last year, the company received FDA approval to market Rezdiffra, or resmetirom, as an orally dosed tablet to treat NASH. This is an important advance for both doctors and patients, as Rezdiffra is the first approved medication for NASH that does not require a liver biopsy to confirm the diagnosis before prescribing.
Getting an approved medication onto the market is the end goal of every research-oriented biopharmaceutical company, as it can mean the difference between long-term success and instant failure. Madrigal launched Rezdiffra in April of last year, following the FDA approval, and has seen revenues increase with each quarter since. In the last reported financial results, for 4Q24 and the full-year 2024, the company reported Rezdiffra sales of $103.3 million for the quarter and $180.1 million for the year. By the end of 2024, the company was able to report that there were more than 11,800 patients currently taking Rezdiffra by prescription.
In addition to the successful launch of its first approved drug, Madrigal was able to finish 2024 with a solid piece of good news from the clinical trial front. The company reported positive two-year data from MAESTRO-NAFLD-1, a double-blind, placebo-controlled, randomized Phase 3 safety trial that was designed and conducted specifically to support the regulatory approval of Rezdiffra. The data showed a statistically significant reduction in liver stiffness, an important indicator of reduced progression to the end stages of liver disease, as well as a reiteration of the drug’s acceptable safety and tolerability profile.
The new drug launch is the cornerstone of Andrea Newkirk’s write-up on Madrigal for Goldman. The biotech expert says of the company and the success of its first new drug, “Through the first nine months of launch, Rezdiffra continues tracking well against top-tier specialty drug launches, which we expect to drive upwards earnings revision throughout 2025. Importantly, now almost one year since approval, MDGL’s growing foothold in the commercial NASH market reflects success in establishing a pathway (from education to treatment to access) and positions Rezdiffra favorably in the face of an evolving competitive landscape, particularly on the back of growing evidence of Rezdiffra’s therapeutic benefit in F4 cirrhotic NASH patients, which bodes well ahead of the MAESTRO-NASH Outcomes data in 2027. Net, we see MDGL as poised for one of the most successful early launch stories this year and reiterate our Buy rating.”
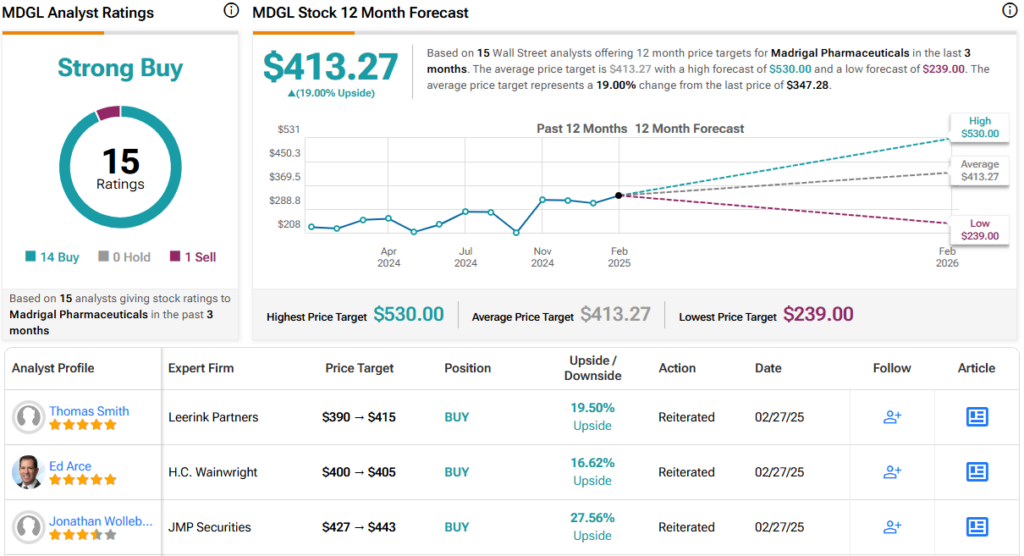
Along with that Buy rating, Newkirk sets a $539 price target, pointing to a one-year gain of 55%.
Overall, this stock has a Strong Buy consensus rating, based on 15 recent analyst reviews that include 14 to Buy against 1 to Sell. The shares are priced at $347.28, and the $413.27 average price target suggests a one-year upside potential of 19%. (See MDGL stock forecast)
To find good ideas for healthcare stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.
















