Cara Therapeutics (CARA) is on an uptrend again, with recent news of the U.S. Food and Drug Administration’s (FDA) approval of the company’s KORSUVA injection. The drug is for treating moderate-to-severe pruritus, occurring in chronic kidney disease (CKD) patients.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
The approval comes after two phase-III trials with positive results. The company recently released its Q3-2021 report, which included a revenue surprise of $20 million, as well as insight into its go-to-market strategy for KORSUVA. The company’s stock price is up around 26% over the last six months. (See Cara Therapeutics stock charts on TipRanks)
I rate the company as bullish. (See Analysts’ Top Stocks on TipRanks)
Last April, the company’s stock hit its all-time high of $29.65, and then fell to a new all-time low of $11.22. It is now back on an uptrend, with good reason to believe that the company’s valuation is hitting a new cycle of growth. The company has promising results for its current research pipeline. It expects benchmark payments and new revenue streams soon from the sale of KORSUVA.
FDA Approval of KORSUVA
The FDA approved the company’s KORSUVA injection, which is used to treat the side effects experienced on the skin from hemodialysis treatment, a type of kidney dialysis. The condition is called pruritus (dry itchy skin) and it can become quite severe in the CKD patient.
The drug is an opioid receptor agonist which acts on the peripheral nervous system and brings meaningful relief to the symptoms. It is the first and only approved treatment for pruritus associated with CKD disease and hemodialysis.
Cara Therapeutics will bring the drug to the U.S. market through a 60/40 partnership with Vifor Pharma. The launch will occur during Q1-2022. The company and Vifor have an exclusive license for the commercialization of KORSUVA injection.
Through a subsidiary of Vifor, the company is in an agreement to seek approval and bring the drug to market in the European Union. The drug is currently being reviewed by the European Medicines Agency (EMA) and a decision is expected during Q2-2022.
Cara Therapeutics received $50 million in Vifor common stock as part of the partnership, and Cara Therapeutics stands to receive up to $240 million upon the achievement of certain sales milestones. From an earlier agreement with Vifor, Cara received a $15 million cash payment from Vifor, based on an FDA approval and a $5 million milestone payment.
The $20 million Q3 revenue surprise showed an increase of 118% compared to last year’s quarter. The company typically reports low revenue numbers per quarter, since it has no products on the market. The company expects this to change as the new drug becomes available in the U.S. and in the EU. Accordingly, the company’s valuation will begin to grow as it moves to the revenue stage.
The company has additional research and treatment applications underway for KORSUVA. It is conducting clinical research which looks at the effectiveness of oral KORSUVA in the treatment of atopic dermatitis and stage 4 pre-dialysis CKD patients. These studies will occur during Q1-2022. The drug is likely to be approved for additional medical applications.
Q3-2021 Results
The company reported revenue of $20 million for Q3-2021. It reported a net loss of $1 million and cash on hand of $193.4 million. Its research and development expenses were $14.4 million.
The company explains in its Q3 discussion that it has enough cash to complete all research through 2023 without additional milestone payments or revenues. The company is well-positioned to fulfill its research agenda, bring the drugs to market, and expand its market reach.
Wall Street’s Take
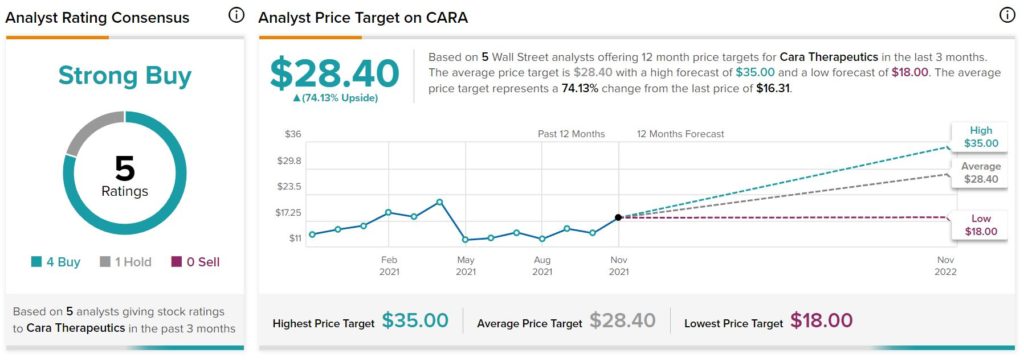
According to Wall Street, Cara Therapeutics has a Strong Buy consensus rating, based on four Buys and one Hold assigned in the past three months. At $28.40, the average Cara Therapeutics price target implies 74.1% upside potential.
Conclusion
In conclusion, I rate Cara Therapeutics as bullish because of the FDA approval of its drug, the company’s go-to-market strategy, and the stock’s price uptrend. The company has firm plans for a U.S. release of its new drug, and a strategy for a European release.
Cara Therapeutics has additional research in progress which will expand the drug’s applications and market reach. In addition, it has plenty of assets to complete its current research pipeline and bring the drug to market. As a result, investors should watch developments from this company and its stock price performance.
Disclosure: At the time of publication, Alan Sumler had no position in Cara Therapeutics.
Disclaimer: The information contained in this article represents the views and opinion of the writer only, and not the views or opinion of Tipranks or its affiliates, and should be considered for informational purposes only. Tipranks makes no warranties about the completeness, accuracy or reliability of such information. Nothing in this article should be taken as a recommendation or solicitation to purchase or sell securities. Nothing in the article constitutes legal, professional, investment and/or financial advice and/or takes into account the specific needs and/or requirements of an individual, nor does any information in the article constitute a comprehensive or complete statement of the matters or subject discussed therein. Tipranks and its affiliates disclaim all liability or responsibility with respect to the content of the article, and any action taken upon the information in the article is at your own and sole risk. The link to this article does not constitute an endorsement or recommendation by Tipranks or its affiliates. Past performance is not indicative of future results, prices or performance.









