The share price of gene-therapy pioneer bluebird bio (BLUE) has come under intense selling pressure over the past few weeks. The reason for the sell-off is that a Sickle-Cell Disease (SCD) patient received an initial Lentiglobin (BLUE’s lead product) treatment and has since developed Acute Myeloid Leukemia (AML).
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Lentiglobin is utilized to treat a host of blood abnormalities. The product is marketed in Europe under the trademark Zynteglo to manage beta-thalassemia, a rare inherited disorder where a patient’s blood lacks sufficient hemoglobin. The lack of adequate hemoglobin leads to fatigue and other more life-threatening conditions.
The development of AML by the SCD patient set off a chain reaction within BLUE, halting all further testing and marketing of its lead product until the origin of the AML case could be determined.
SCD affects the hemoglobin levels in patients as the red blood cells that carry oxygen throughout the body distort to become crescent shaped. SCD patients are prone to vascular sticking and fatigue as hemoglobin levels are often far less than optimal. These patients are also more vulnerable to various blood cancers such as AML.
Since the suspension of the clinical trials evaluating Lentiglobin, BLUE has been thoroughly investigating the root cause of the AML diagnosis, as a clinical failure would more than likely spell doom for the product.
Initial evidence indicated that the BB305 lentiviral vector used in the treatment of the patient was present in his AML cells. However, BLUE announced on March 10 that contrary to initial views, the BB305 lentiviral vector was found in the patient’s VAMP4 gene, which has zero correlation to the development of AML. This data was validated using historical medical literature in over 700 human cell lines.
BLUE added further evidence via laboratory analysis, indicating that the AML patient had significant pre-existing chromosomal abnormalities that aided in the formation of AML. The patient’s sample also showed abnormal mutations in the RUNX1 and PTPN11 genes, the most likely culprits in his development of AML.
Risks To The Path Forward
BLUE bounced a little on the release of the positive data regarding the AML case, but shares are still trading far below the $44 level seen prior to the clinical hold. The company’s lead product still carries significant skepticism, which could offer aggressive investors a potentially risky, but highly rewarding scenario.
If the clinical hold is lifted based on the evidence gathered during the investigations, the marketing of Zynteglo should re-commence once again, and BLUE could be a bargain at current levels.
However, investors need to be comfortable with some of the key risks inherent in the stock, as there are no guarantees that the regulatory commissions will allow the clinical trials to resume. The FDA has become more hostile of late with gene expression companies such as BLUE due to the high costs of treatment. The FDA may require further data, delaying the product’s entry into the market.
Secondly, the SCD field has grown more crowded, with some prominent companies offering a competitive product. If practitioners view the Lentiglobin therapy as tainted, they may opt for a newer treatment once it reaches the market, thereby denying BLUE the long-term, annuity-like revenue stream that a blockbuster product could bestow.
What Does Wall Street Think About BLUE?
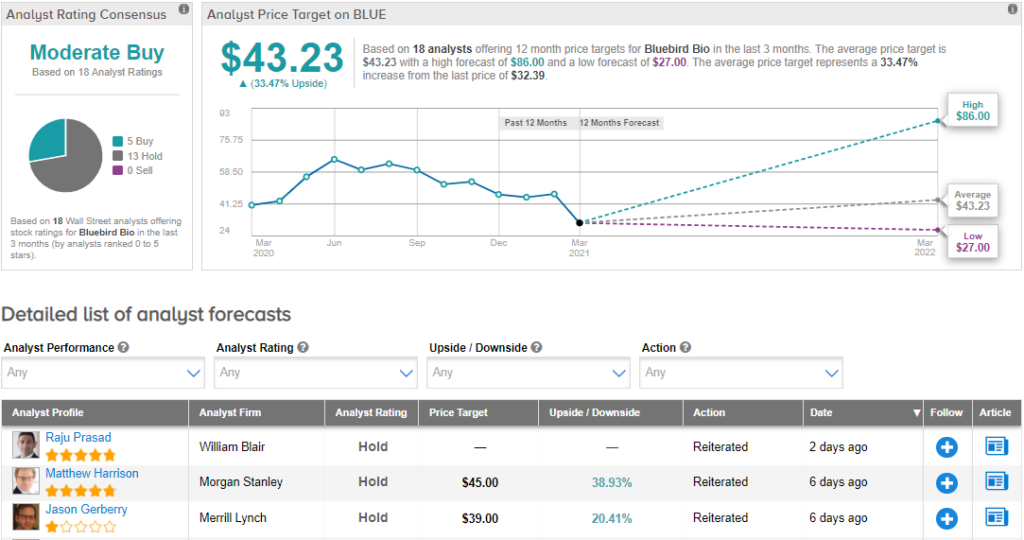
Bluebird Bio receives a Moderate Buy consensus rating among Wall Street analysts. Of the 18 analysts who assigned a rating to the stock, 5 recommend a Buy and 13 suggest a Hold. The average analyst price target of $43.23 implies that BLUE has around 33% upside potential from current levels over the next 12 months. (See bluebird bio stock analysis on TipRanks)
Disclosure: Alexander Poulos was long BLUE at the time of publication.
Disclaimer: The information contained herein is for informational purposes only. Nothing in this article should be taken as a solicitation to purchase or sell securities.









