Biogen (NASDAQ:BIIB) agreed to pay $900 million to resolve claims that it paid kickbacks to doctors for prescribing its drugs over competitors. Though Biogen denied all allegations raised by its former employee, Michael Bawduniak, in this case, it agreed to resolve the matter to focus on its strategic priorities, the company stated.
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
Notably, during the Q2 conference call, its CFO Mike McDonnell said, “we recorded $900 million, plus estimated fees and expenses” charge to “resolve a previously disclosed qui tam litigation relating to conduct prior to 2015.”
However, he added that this “does not include any admission of liability.”
Besides this litigation setback, Biogen lost the ADUHELM (Alzheimer’s disease drug) opportunity earlier this year. The Centers for Medicare & Medicaid Services restricted ADUHELM coverage to clinical trials. The move significantly reduced the commercial potential for ADUHELM.
Is Biogen a Good Investment?
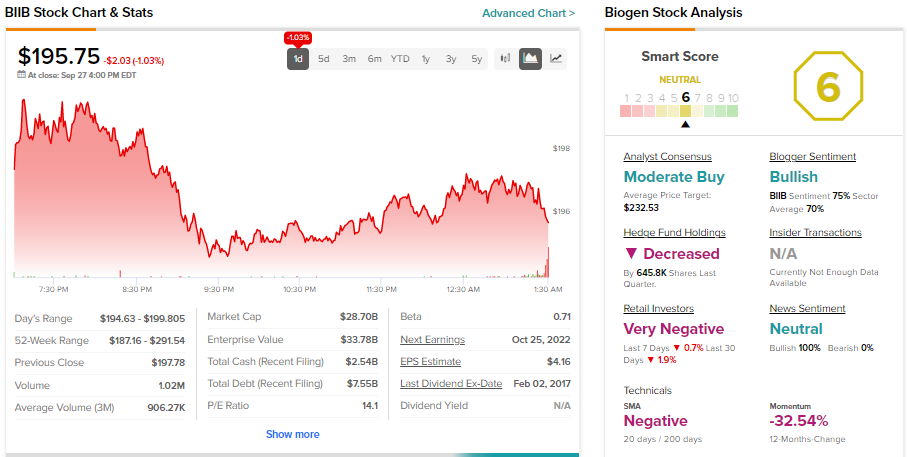
With back-to-back setbacks, it’s prudent to ask whether BIIB is a good investment. TipRanks’ data shows that Wall Street analysts remain sidelined on BIIB stock. It has received nine Buys and nine Holds for Hold consensus rating. Further, analysts’ average price target of $232.53 implies 18.8% upside potential.
While analysts remained sidelined, hedge funds and retail investors reduced their exposure to BIIB stock. Hedge funds sold 645.8K BIIB shares last quarter. Meanwhile, TipRanks’ data shows retail investors lowered exposure to BIIB stock by 1.9% in 30 days.
Overall, BIIB has a Neutral Smart Score of six out of 10 on TipRanks.
Bottom Line
The setbacks from litigation and ADUHELM and increased competitive activity remain a drag on BIIB stock. A lot would depend on new product launches and positive outcomes for its second Alzheimer’s drug, Lecanemab.
Read full Disclosure



















