Shares in Apyx Medical Corp. (APYX) spiked 45% in pre-market trading after the medical devices manufacturer won regulatory approval to sell its Helium Plasma technology products in five new countries.
The stock leaped to $6.93. Apyx received approval to sell its Helium Plasma technology products in Australia, Brazil, Israel, Taiwan and Thailand. The company said it has contracted with distributors to market its products in each country. The products are sold as Renuvion in the cosmetic surgery market and J-Plasma in the hospital surgical market.
Renuvion, which is used for cosmetic medical procedures such as dermal resurfacing, is a device using the helium technology to apply energy beneath the skin, causing a contraction effect that’s gentle on tissue. J-Plasma is a device used in cosmetic surgery for cutting, coagulation, and ablation of tissue.
“We have been focused on pursuing regulatory clearances in international markets as one of our key strategic priorities to enhance Apyx Medical’s long-term growth profile,” said Apyx CEO and President Charlie Goodwin. “This strategy is based on the prudent expansion of our commercial footprint outside the U.S. by securing the requisite product registrations for our Helium Plasma Technology products, and identifying strong distribution partners in each respective market.”
Goodwin added that he sees great growth opportunity in Brazil, which is estimated to be the second largest cosmetic surgery market in the world in terms of both the total number of procedures performed and the number of surgeons.
“We shipped an initial commercial order to our distributor in Brazil in late-June and we expect to ship our initial commercial orders to each of the other new countries over the second half of 2020,” Goodwin said.
Shares in Apyx have been hit hard this year, plunging some 43%. The stock rose 2.1% to $4.78 as of Monday’s close.
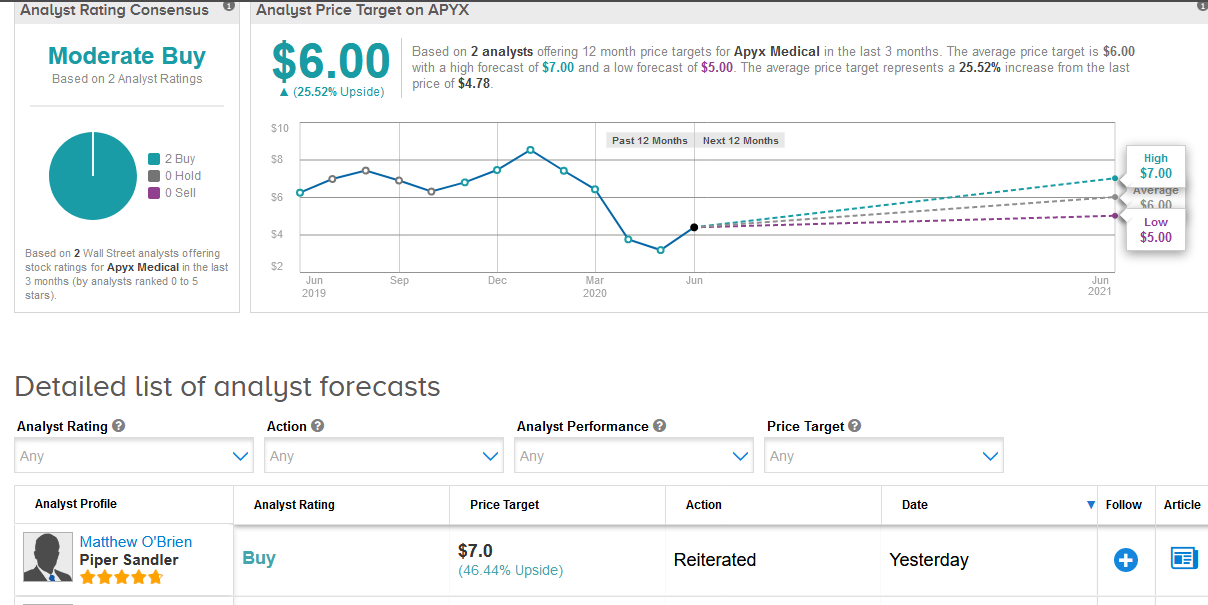
Five-star analyst Matthew O’Brien at Piper Sandler maintained a Buy rating on the stock with a $7 price target, saying that the regulatory approval is an “important step” for building out the company’s international customer base and should generate a “nice chunk” of growth in the coming years.
O’Brien added that although Apyx’s shares are still down significantly year-to-date, he believes the company remains in sound financial shape and he expects the stock to recover as the coronavirus pandemic begins to subside.
Related News:
Merck’s Gardasil Receives FDA Nod For Expanded Cancer Indications
Evoke Pharma Pops 86% On FDA Approval Of Gimoti Nasal Spray
Sanofi, Regeneron Win China Approval For Dupixent Dermatitis Drug