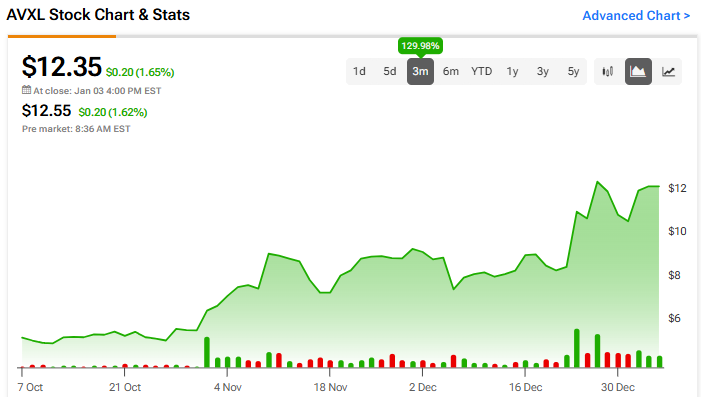
Anavex Life Sciences (AVXL) finished 2024 with its stock surging roughly 130% in the last three months. It was catalyzed by news of significant progress with ANAVEX 2-73, its pioneering drug candidate for Alzheimer’s disease. The treatment has shown potential in slowing cognitive and functional decline and offers a robust safety profile, paving the way for alternatives in the Alzheimer’s treatment realm. With the European Medicines Agency (EMA) now accepting the application for treatment of early Alzheimer’s, the company could see a market launch as early as 2025. Despite inherent risks such as regulatory challenges, competition, and financial constraints, the company’s risk-reward profile marks it as an attractive option for biotech investors.
A Potential Blockbuster Drug in the Pipeline
Anavex Life Sciences is a biopharmaceutical company that focuses on developing therapeutic solutions for neurodegenerative, neurodevelopmental, and neuropsychiatric conditions like Alzheimer’s, Parkinson’s, and schizophrenia. The company’s leading drug candidate, ANAVEX 2-73 (blarcamesine), has successfully completed Phase 2a and Phase 2b/3 clinical trials for Alzheimer’s treatment, a Phase 2 study in Parkinson’s disease dementia, and Phase 2/3 trials for adult and pediatric patients with Rett syndrome.
ANAVEX 2-73 is an orally administered drug intended to reinstate cellular homeostasis by engaging with SIGMAR1 and muscarinic receptors. In preclinical studies, it’s been seen to potentially stop or reverse Alzheimer’s disease progression. It also demonstrated a range of beneficial properties, suggesting it could be effective for other CNS conditions, like epilepsy.
The European Medicines Agency (EMA) has accepted the Marketing Authorization Application (MAA) for review to treat Alzheimer’s disease, which affects an estimated seven million individuals in Europe. This marks a key step in potential commercialization for ANAVEX 2-73.
ANAVEX 3-71 has also shown promise in clinical stages, demonstrating activity against major Alzheimer’s disease markers in transgenic mice. It has exhibited significant benefits for mitochondrial dysfunction and neuroinflammation.
A Four-Year Runway
For Q4 2024, the company reported a net loss of $11.6 million, or $0.14 per share, an increase from the $10.1 million, or $0.12 per share, seen in the equivalent quarter of the previous fiscal year. This was driven by increased Research and Development expenditures, from $10.1 million in Q4 2023 to $11.6 million. Also, General and Administrative expenses rose slightly from $2.6 million in Fiscal 2023 to $2.8 million.
As of the end of September 2024, the company reported cash and cash equivalents amounting to $132.2 million, experiencing a decrease from the $151 million in the same period the previous year. Despite this reduction, the company expects sufficient funds to maintain its operations at the current cash utilization rate for approximately four more years.
Tremendous Upside Potential
The stock has been on an upward trend, climbing 88% over the past year. It trades near the high end of its 52-week price range of $3.25 – $14.44 and shows ongoing positive price momentum as it trades above major moving averages.
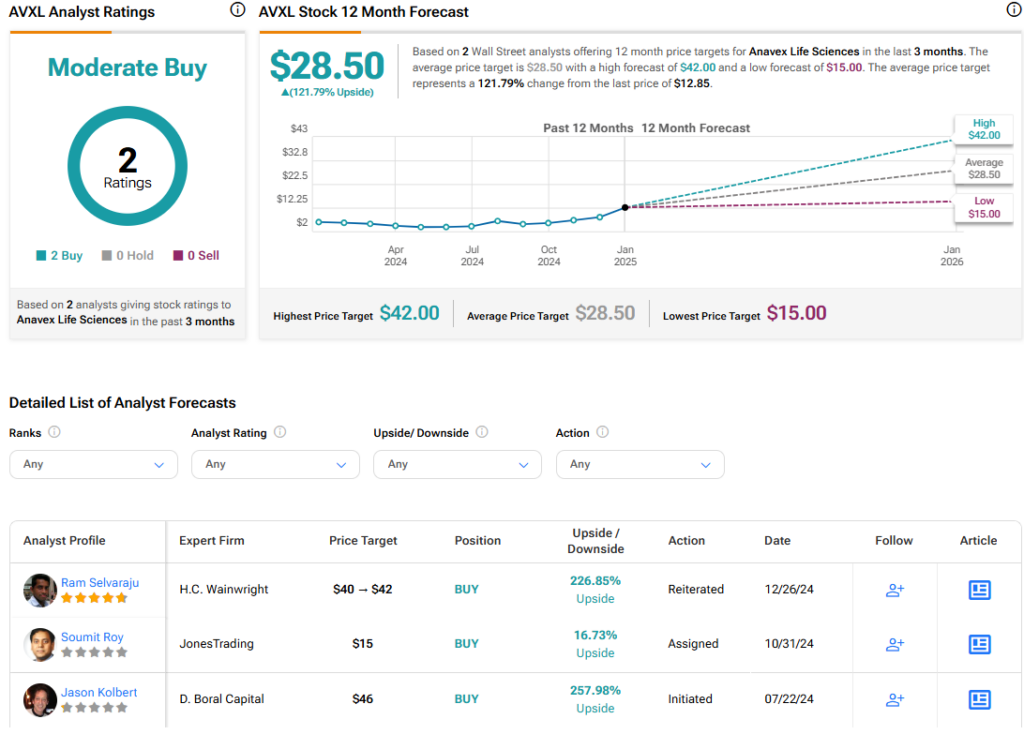
Analysts following the company have been constructive on AVLX stock. For instance, H.C. Wainwright analyst Ram Selvaraju, a five-star analyst according to Tipranks’ ratings, recently raised the price target on the shares to $42 (from $40) and maintained a Buy rating after the company announced the EMA acceptance of the Marketing Authorization Application for treating Alzheimer’s disease.
Anavex Life Sciences is rated a Moderate Buy overall, based on two analysts’ recent recommendations. Their average price target for AVXL stock is $28.50, which represents a potential upside of 130.77% from current levels.
Final Analysis on AVXL
Anavex Life Sciences has wrapped up 2024 on a promising note, with its shares surging on news of significant strides in the development of ANAVEX 2-73. This pioneering drug has shown promise in halting cognitive decline. With the European Medicines Agency now assessing the application for the treatment of early Alzheimer’s, a market launch could be on the horizon as soon as 2025. Moreover, other impressive pursuits, like treatments for schizophrenia, are also underway. While obstacles such as regulatory challenges, competition, and financial constraints are always to be considered, the risk-reward profile of AVXL makes it an enticing proposition for biotech investors.
















