Shares in Acadia Pharmaceuticals (ACAD) plunged 12% in Monday’s extended trading, after the company announced disappointing top-line results from its 298 patient Phase 3 CLARITY study evaluating pimavanserin as an adjunctive treatment for major depressive disorder (MDD).
Don't Miss Our Christmas Offers:
- Discover the latest stocks recommended by top Wall Street analysts, all in one place with Analyst Top Stocks
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
The study did not achieve statistical significance on the primary endpoint which was the 17-item Hamilton Depression Rating Scale (HAMD-17) total score change from baseline to week 5. Pimavanserin 34 mg, given once-daily as an adjunctive treatment to standard antidepressant therapy was associated with a mean reduction of 9.0 in HAMD-17 total score compared to 8.1 for placebo as an adjunctive treatment (p=0.296).
Positive results were observed on the key secondary endpoint, the Clinical Global Impression – Severity (CGI-S) score, a clinician assessment of a patient’s severity of depression (nominal p=0.042) and on the Karolinska Sleepiness Scale (KSS) score (nominal p=0.005).
“We observed a consistent improvement of depressive symptoms over time with pimavanserin but, unfortunately, the robust positive results from our CLARITY-1 study were not replicated,” said Serge Stankovic, ACADIA’s President. “While these results do not support the product profile to pursue an additional Phase 3 study in adjunctive MDD, we will continue to analyze the data and the findings from our earlier positive depression studies as we assess next steps.”
In the study, pimavanserin was generally well-tolerated when added to existing antidepressant therapy, and similar rates of adverse events were observed between pimavanserin (58.1%) and placebo (54.7%).
However, on a more positive note, the company also announced that the FDA accepted Nuplazid’s (pimavanserin) supplemental new drug application (sNDA) for dementia-related psychosis (DRP). The PDUFA date for the FDA’s decision is set for April 3, 2021.
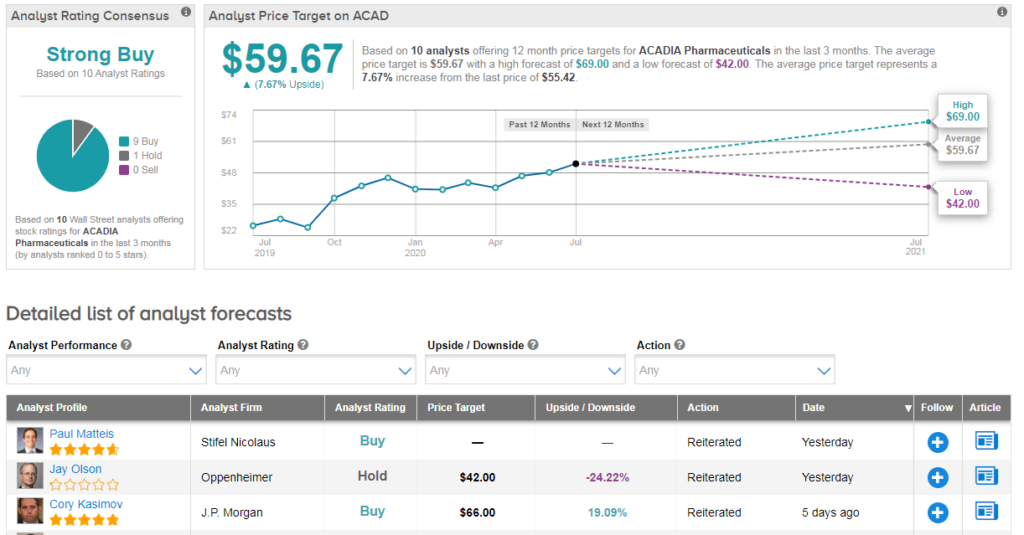
“Nuplazid’s Phase 3 failure in major depressive disorder (MDD) is certainly disappointing but not entirely surprising given how difficult a nut this indication is to crack” commented JP Morgan analyst Cory Kasimov following the update.
However he remains bullish on ACAD’s longer-term outlook and the DRP indication specifically. “We remain highly confident in the approvability of DRP” the analyst commented. He has a buy rating on the stock and $66 price target (19% upside potential).
Shares in ACAD are up 30% year-to-date, and the stock shows a bullish Strong Buy analyst consensus. That’s with an average analyst price target of $60 (8% upside potential). (See ACAD stock analysis on TipRanks)
Related News:
Pfizer, BioNTech Ink UK Supply Deal For 30M Covid-19 Vaccine Doses
NuVasive Spikes 5% After-Hours On Sharp Procedure Rebound
GSK Buys 10% Stake In Germany’s CureVac To Develop mRNA Vaccines



















