AbbVie announced that it has signed an exclusive global collaboration agreement with I-Mab to develop and sell the Chinese biotech company’s lemzoparlimab drug candidate for the treatment of multiple cancers.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Under the terms of the agreement, AbbVie (ABBV) will pay I-Mab $180 million in an upfront payment to exclusively license lemzoparlimab, also known as TJC4, along with $20 million in a milestone payment based on the Phase 1 results, for a total of $200 million. I-Mab will be eligible to receive up to $1.74 billion in success-based milestone payments for lemzoparlimab, of which $840 million are based on clinical development and regulatory approval milestones, with the remainder based on commercial milestones.
Upon commercialization of lemzoparlimab, AbbVie will also pay tiered royalties from low-to-mid teen percentages on global net sales outside of greater China. In addition, the two partners have the potential to expand the collaboration to additional transformative therapies, they said in the joint statement.
Nasdaq-listed I-Mab (IMAB) stock closed 3.6% higher at $37.07 on Friday, while AbbVie was little changed.
Lemzoparlimab is one of the main drug candidates among I-Mab’s proprietary and innovative pipeline. It is designed to minimize inherent binding to normal red blood cells while preserving its strong anti-tumor activity, a critical attribute in potentially differentiating lemzoparlimab from other antibodies of the same class currently in development, the company said.
“Cancer is the second-leading cause of death globally and the need for novel cancer therapies has never been more acute. The addition of I-Mab’s novel CD47 programs complements our global clinical strategy in hematology and immuno-oncology,” said AbbVie’s chief scientific officer Thomas J. Hudson. “We have been impressed with what I-Mab has been able to accomplish in research and clinical development and we look forward to working together to make a meaningful difference in the lives of millions of patients globally.”
I-Mab said results of its recent phase 1 clinical trial confirm possible differentiation of lemzoparlimab in drug safety and a more favorable pharmacokinetics profile in cancer patients. Results have shown that lemzoparlimab is well tolerated as a single agent at a dose range of up to 30 mg/kg without any priming dose. Full data will be presented at an appropriate scientific conference later this year.
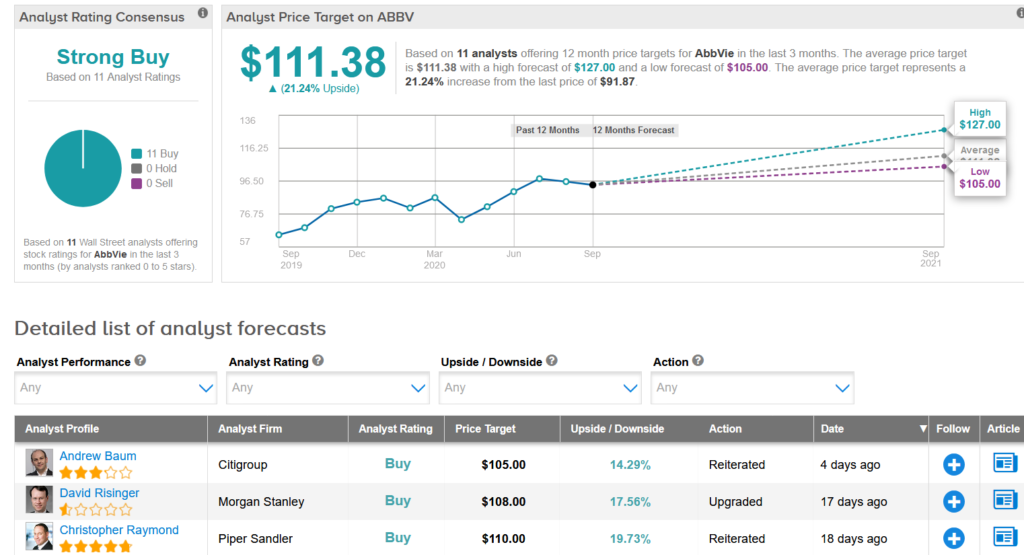
AbbVie shares have recovered after hitting a low in March and are now trading 3.7% higher than at the start of the year on the back of its acquisition of Botox maker Allergan and solid revenues of Humira. Looking ahead, the $111.38 average analyst price target indicates another 21% upside potential in the coming 12 months.
Before the collaboration announcement, Citigroup analyst Andrew Baum last week raised the stock’s price target to $105 from $98 and reiterated a Buy rating.
Baum believes that the “underappreciated revenue and/or durability” upside from the company’s marketed agents Skyrizi, Rinvoq and Imbruvica offsets the current risk from the recently announced congressional subpoena on AbbVie’s drug pricing practices.
Overall, ABBV scores 11 Buy ratings from Wall Street analysts adding up to a bullish Strong Buy consensus. (See Abbvie’s stock analysis on TipRanks).
Related News:
Novavax Pops 8% On Covid-19 Vaccine Data; Analyst Says Buy Amid Pullback
Medtronic’s First-Of-Its Kind Diabetes System For Young Children Approved
AstraZeneca Rises On Report Trump Could Fast-Track Covid-19 Vaccine Candidate