Diversified life sciences company Tauriga Sciences, Inc. (TAUG) has a number of products and product lines that are focused on cannabidiol (CBD) and cannabigerol (CBG) edibles market segment.
Don't Miss Our Christmas Offers:
- Discover the latest stocks recommended by top Wall Street analysts, all in one place with Analyst Top Stocks
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
Its chief product line, Tauri-Gum, is CBD chewing gum. The company also offers anti-nausea and skincare products. On July 12, TAUG launched two new products, a CBD-infused sunscreen spray and a moisturizing lip balm.
Let’s take a look at the company’s financial performance and what has changed in its key risk factors that investors should know.
Tauriga Sciences Risk Factors
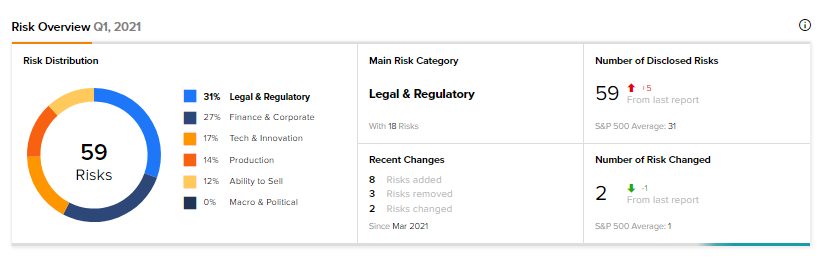
According to the new Tipranks Risk Factors tool, Tauriga’s main risk category is Legal & Regulatory, which accounts for 31% of the total 59 risks identified. The next two major risk factor contributors are Finance & Corporate, and Tech & Innovation at 27% and 17%, respectively. Since March, the company has added eight new risk factors and removed three.
Let’s have a look at the key new risk factors added by the company.
Under Tech & innovation category, the company acknowledges that for a healthcare firm, clinical drug development is a critical process. This can be a lengthy and expensive process with an uncertain outcome. Any delay in the drug development process means potential additional costs. A failure of one or multiple clinical trials can happen at any stage of product development.
These delays, or unforeseen events, such as adverse trial participant reactions can lead to additional trials, negative results, or abandonment of a product development program.
Under the Legal & Regulatory risk category, Tauriga notes that the legal definition of ‘synthetic cannabinoids’ is constantly evolving and a regulatory uncertainty remains over the potential classification of these products. Significantly, several states in the U.S. have banned the use of industrial hemp-derived Delta-8-Tetrahydrocannabinol (Δ8 THC) in consumer products.
Proposed regulations in this regard are currently in a public comment period and a cloud of uncertainty hangs over what the final regulations will include.
Under the Production risk category, Tauriga highlights that members of its management team lack experience in the pharmaceutical field. Tauriga will need to hire or engage experts with relevant experience in the field to maintain an effective management team with experience in commercializing pharma products. A failure in this regard may have an adverse impact on the business.
Finally, Tauriga relies on third parties to conduct its pre-clinical and clinical trials. If these parties do not perform as required, or expected, it may hamper Tauriga’s product development and commercialization efforts.
Financial Performance
Amid the COVID-19 pandemic, Tauriga’s distributor sales evaporated from $62.4 thousand in fiscal 2020 to none in fiscal 2021. Wholesale sales too dropped to $51.3 thousand in fiscal 2021 from $137.5 thousand a year ago.
Despite these challenges, focus on E-commerce sales, along with marketing initiatives and new product launches, helped its online sales jump to $234 thousand from $34.4 thousand a year ago. This helped the company’s total revenue to increase to $285.3 thousand in fiscal 2021 from $234.4 thousand in fiscal 2020.
Meanwhile, a simultaneous jump in marketing and R&D spending in fiscal 2021 widened the company’s net loss to $3.39 million versus a net loss of $2.1 million in fiscal 2020. (See Tauriga Sciences stock chart on TipRanks)
Bottom Line
The Legal & Regulatory risk factor’s sector average is at 20%, compared to Tauriga’s 31%. Shares have gained 40% over the past year.
Related News:
Accenture Concludes Purchase of Linkbynet
What Do VistaGen’s Newly Added Risk Factors Mean for Investors
What Do AMMO’s Newly Added Risk Factors Mean for Investors



















