Shares of diagnostics solutions provider LumiraDX (NASDAQ:LMDX) are up nearly 33% at the time of writing today after its SARS-CoV-2 Ag Ultra and SARS-CoV2 Ag and RSV tests bagged validation for use under the Coronavirus Test Device Approvals (CTDA) mechanism in the U.K.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
Impressively, these tests enable differentiation between SARS-CoV-2 and RSV amid respiratory symptoms and come ahead of the respiratory season in the U.K. Moreover, the SARS-CoV-2 Ag Ultra helps detect SARS-CoV-2 in only five minutes and can help improve clinical workflows.
The company has already launched the SARS-CoV-2 Ag Ultra assay in the European Union as well as other markets. It now expects to launch these tests in the U.K. and begin shipments imminently.
David Walton, the Chief Commercial Officer at LumiraDX commented, “By providing rapid, high sensitivity point of care tests for COVID-19, Flu, and RSV, we hope to reduce the burden of respiratory illness, facilitate clinical workflows and improve patient outcomes.”
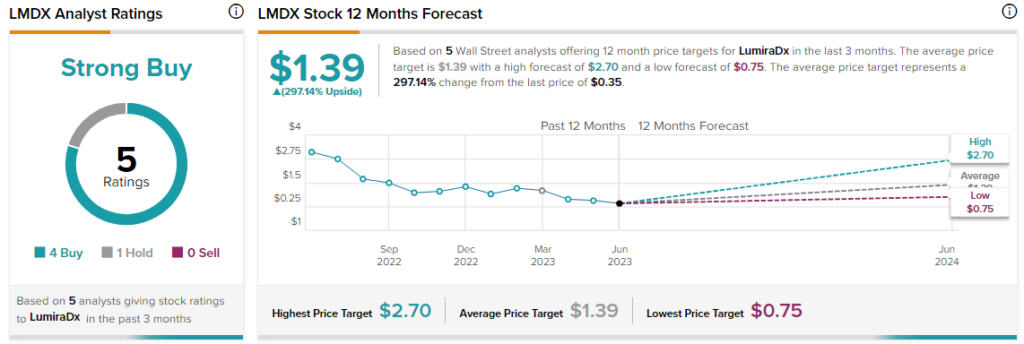
Overall, the Street has a $1.39 consensus price target on LumiraDX alongside a Strong Buy consensus rating.
Read full Disclosure



















